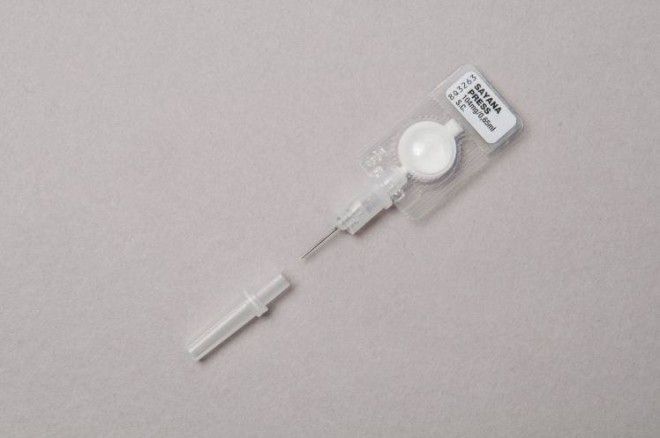
“This is an exciting milestone for women in the United Kingdom, and, potentially, in countries around the world, who might prefer this method of contraception and mode of administration,” said Dr. Salomon Azoulay, senior vice president and chief medical officer at Pfizer, in a statment. “With this revised label, following consent from a healthcare professional and with proper training, UK women will now have the opportunity to administer Sayana Press outside of a clinical setting.”
Each jab comes in a single-use, non-reusable injection system, eliminating the need for preparing needles and syringes. According to Pfizer, each injection should last around 13 weeks, plus or minus one week, with women who opt for its use to be taught how to safely inject the drug by a doctor or nurse first. They will then be required to see a doctor once a year to check everything is ok.
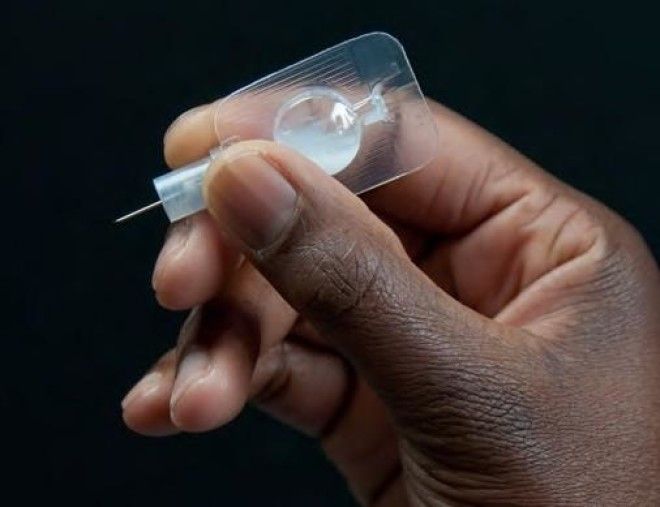
“When it comes to contraception, women may require different options that suit them at different times in their lives,” Seema Patel, a medical director at Pfizer, told. “We appreciate that many women are very busy and that visiting their healthcare professional regularly to pick up their contraception can be a challenge.”
The company is currently seeking approval for other European countries, as well as continuing to roll it out to the rest of the world, especially in developing nations. Last year, Pfizer signed an agreement with both the Gates Foundation and the Children’s Investment Fund Foundation to make the injections available in 69 of the world’s poorest countries for just $1 a dose.

